3D Medicines Completed Follow-Up for the First Patient in Global Exclusive Low-Dose RDC Candidate 3D1015 Human Data Trial
Shanghai, China – On October 23, 2025, 3D Medicines (1244.HK) announced the successful completion of follow-up for the first patient enrolled in the investigator-initiated trial (IIT) of 3D1015, its core radioligand drug conjugate (RDC) candidate. The study, designed to evaluate the safety and radiation dosimetry of 3D1015 in patients with metastatic castration-resistant prostate cancer (mCRPC), administered the initial dose on August 27, 2025. The patient has since completed all scheduled follow-up assessments, and the trial is progressing as planned. Preliminary findings indicate robust radiotracer accumulation in target lesions, absence of grade ≥3 drug-related adverse events following a single administration, and a downward trend in prostate-specific antigen (PSA) levels, despite a dose amounting to only 1/20th of that used in approved comparator product (Pluvicto®,177Lu-PSMA-617 ). These early results provide a foundation for further clinical development.
In recent years, RDC technology has emerged as a leading frontier in next-generation oncology due to its ability to deliver targeted radiotherapy with high specificity. The commercial success of Novartis’ Pluvicto® in mCRPC treatment exemplifies the therapeutic and market potential of this modality: global sales reached $1.392 billion in 2024, representing a 42% year-on-year increase. At the 2025 JPMorgan Healthcare Conference, Novartis projected peak annual sales exceeding $5 billion. This trajectory has catalyzed strategic investments by major pharmaceutical companies, including Eli Lilly, Bristol-Myers Squibb (BMS), and AstraZeneca, through high-value acquisitions and collaborative partnerships. Industry analysts estimate the global RDC market will surpass $24 billion by 2030. With over a decade of dedicated research spanning small-molecule inhibitors to radiopharmaceuticals targeting prostate-specific membrane antigen (PSMA), 3D Medicines is uniquely positioned to advance 3D1015 as a novel, low-dose, precision-targeted therapy offering enhanced efficacy and improved safety profiles for mCRPC patients worldwide.
Targeted and Precise Upgrading to Build Core Competitive Barriers
3D1015 targets PSMA and is conjugated to the beta-emitting radionuclide lutetium-177 (¹⁷⁷Lu), incorporating key refinements in molecular design to enhance targeting efficiency. Preclinical data demonstrated that the small-molecule ligand of 3D1015 exhibited a significantly improved binding affinity to PSMA (2 times that of Pluvicto®) and a highly efficient internalization into LNCaP cells (1.5 times that of Pluvicto® ).Notably, tumor tissue exposure to 3D1015 is approximately 3.2 times higher than that of Pluvicto® (Figure 1), while rapid clearance from normal tissues minimizes off-target effects. This pharmacokinetic profile, characterized by high tumor retention and low background distribution, not only improves therapeutic precision and reduces toxicity risks but also supports direct competitive positioning relative to Pluvicto®.
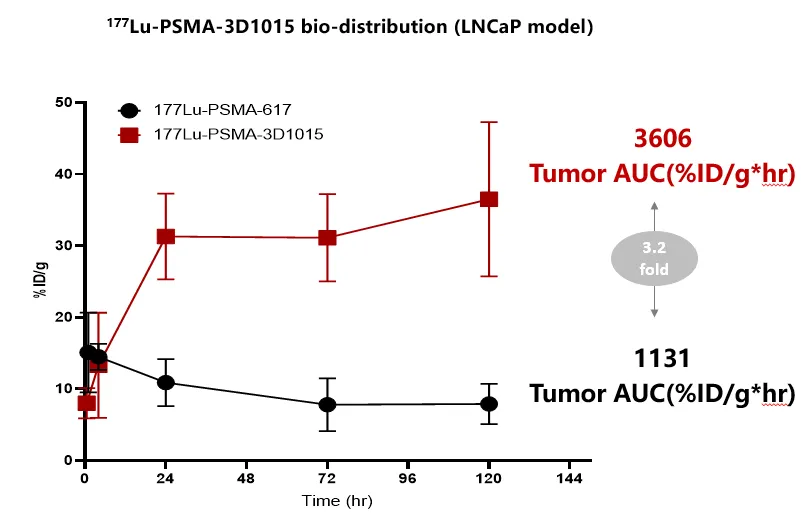
(Figure 1)
Low-dose High Efficacy Laying a Solid Foundation for Commercialization
In preclinical efficacy studies , 3D1015 demonstrated superior dose-efficacy profiles compared to the comparator ( Pluvicto®.) in the LNCaP xenograft mouse model. Data show that the comparator product achieved a tumor growth inhibition rate (TGI) of 94.0% at a dose of 1.0 millicurie (mCi), whereas 3D1015 reached a TGI of 98.3% at 0.5 mCi (equivalent to half the dose of the comparator), demonstrating significant superiority over the comparator at the same dose level. Notably, even when the dose of 3D1015 was reduced to 0.1 mCi, its TGI remained at a relatively high level of 80.0%. Based on these data, we predicts that 3D1015 can achieve comparable tumor inhibitory effects with only approximately one-fourth the dose of the comparator product. (Figure 2) This “low-dose, high-efficacy” paradigm not only reduces patients’ radionuclide exposure, enhances treatment safety, but also lowers demands on radionuclide supply chains and radioactive waste management. Consequently, it facilitates scalable manufacturing, broadens clinical accessibility, and strengthens cost-efficiency, which are key enablers for global commercialization.
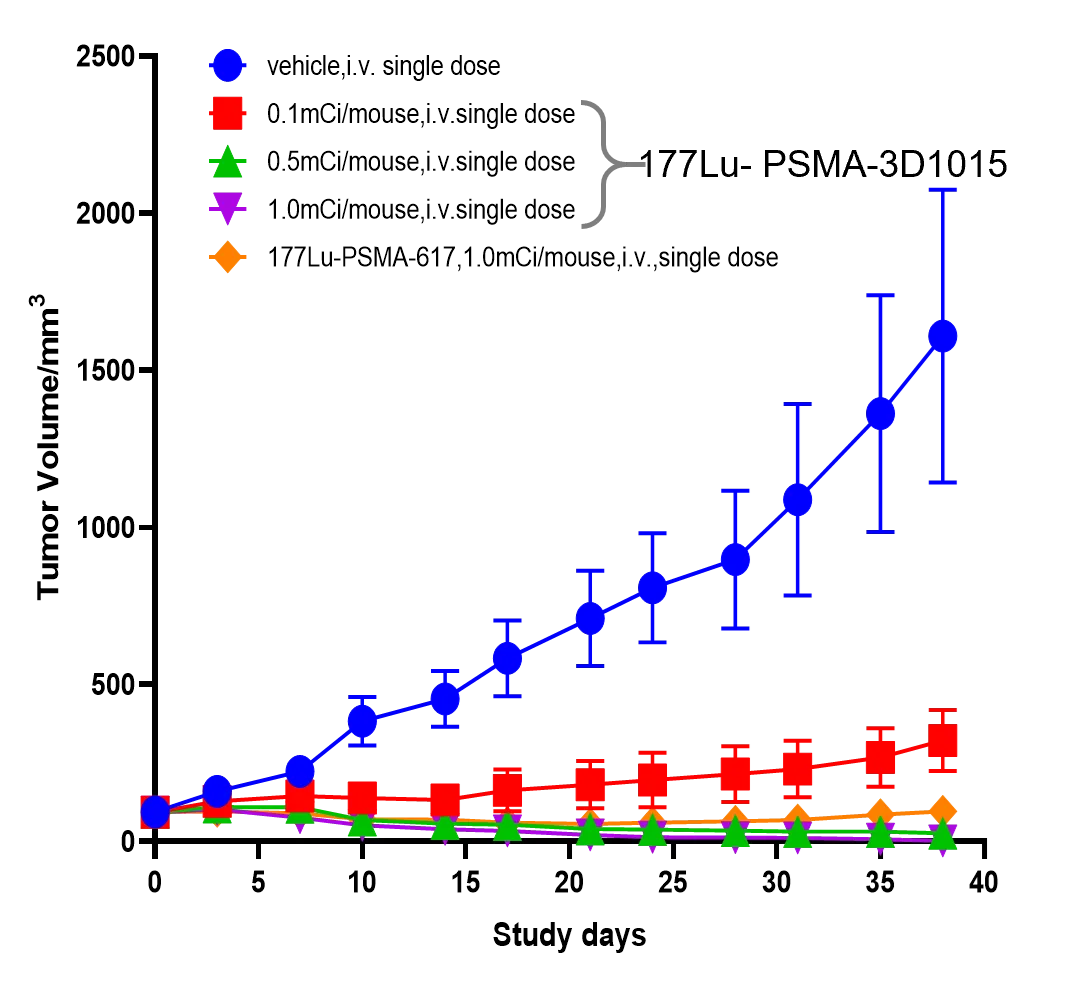
(Figure 2)
Low Toxicity and Favorable Safety Profile Enhancing Patient Benefit Levels
Preclinical toxicity studies demonstrated that 3D1015 exhibited favorable safety and tolerability profiles. In single-dose studies in mice, only a mild decrease in white blood cell (WBC) and lymphocyte (LYM) counts were observed on Day 4 post-administration, with near-complete recovery by Day 29 and no evidence of persistent hematological toxicity. Hepatic and renal function parameters showed no statistically significant differences between treated and control groups. Comprehensive histopathological examinations of major organs—including the heart, liver, spleen, lungs, kidneys, and bone marrow—revealed no obvious treatment-related morphological abnormalities, even at high-dose levels (Figure 3). These findings indicate a minimal organ toxicity burden, which may improve patient compliance, reduce supportive care requirements, and support long-term treatment feasibility—factors critical for real-world adoption and positive patient-reported outcomes.
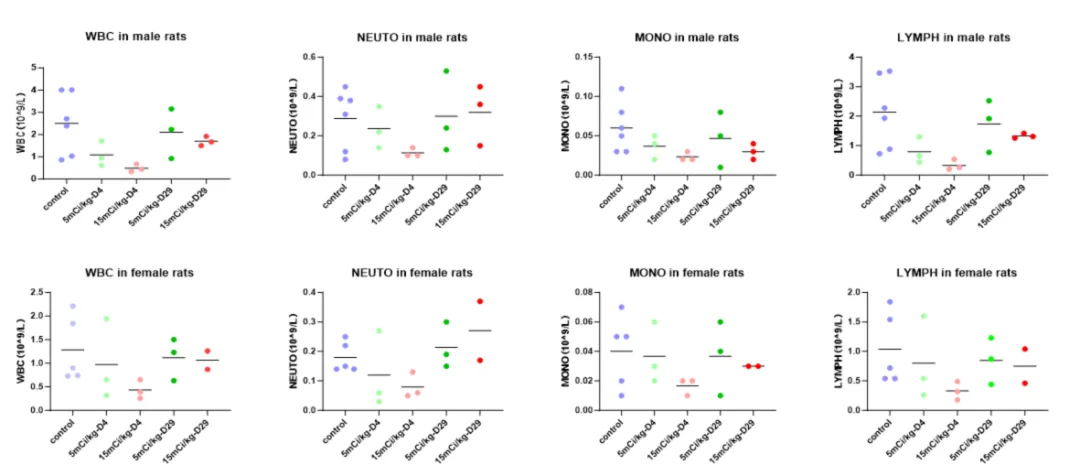
(Figure 3)
Dual Safeguards of Technology and Intellectual Property Empowering Global Competition
The breakthroughs in preclinical data and the progress in clinical translation of 3D1015 are attributed to 3D Medicines' long-term technological accumulation in the field of oncology. The company has established a comprehensive RDC research and development platform system, enabling independent completionof molecular design, screening, and preclinical evaluation, providing core technical support for the research and development of innovative drugs . In terms of intellectual property, we have filed a patent application for 3D1015 with the China National Intellectual Property Administration (CNIPA) and obtained acceptance. Additionally, we have submitted an international patent application via the Patent Cooperation Treaty (PCT) route to conduct a global intellectual property layout. This layout not only ensures the autonomy of the entire chain from research and development to commercialization but also builds a core competitive barrier, providing both legal and technical dual guarantees for its competition in the global radiopharmaceutical market.
As a domestically developed RDC candidate drug in China, 3D1015 is expected to become a next-generation RDC treatment option following Pluvicto® if it can continue to demonstrate its advantages in subsequent clinical studies bringing great benefits to global mCRPC patients in terms of dose optimization, toxicity control, and treatment costs. In the future, 3D Medicines will continue to advance the clinical development of 3D1015, helping domestic radiopharmaceutical products in China to go global and promoting the establishment of an important position for Chinese radiopharmaceutical technology in international competition, bringing more treatment hope to cancer patients worldwide.
About 3D Medicines
3D Medicines Inc. (1244.HK) is a commercial-stage oncology innovative drug research and development company which independently develops multiple globally leading or clinically valuable differentiated innovative drug candidates and tumor vaccine products. It has established an international professional team covering research and development, production, and commercialization, dedicated to helping cancer patients live longer and better. Among its 17 pipelines, 8 have entered the clinical stage. Its first commercial product, 恩维达® (the world's first subcutaneous PD-L1 single-domain antibody), has benefited tens of thousands of patients in the four years since its launch, establishing a differentiated advantage in tumor immunotherapy. Currently, the company drives innovation through three self-developed R&D platforms, including an innovative in vivo CAR-T/NK technology platform, next-generation products of multiple autologous CAR-T cells covering multiple therapeutic scenarios such as tumors and autoimmune diseases, and a breakthrough in traditional ex vivo CAR-T products through an autonomous intellectual property tLNP delivery system, eliminating the need for ex vivo lymphocyte depletion and providing a safer and more accessible off-the-shelf immunotherapy solution; the AI+mRNA tumor vaccine platform, which breaks through patent barriers based on its self-built LNP delivery system, significantly improving mRNA delivery efficiency and reducing toxicity; and the RDC research and development platform for radiopharmaceuticals, focusing on the development of best-in-class potential radiopharmaceuticals. 3D Medicines adheres to the core of patient benefit, through a development strategy that emphasizes both scientific innovation and efficient commercialization, continuously enhancing the global competitiveness of its products, creating long-term value for shareholders, and promoting sustainable growth in performance.
For more information,
please visit http://www.3d-medicines.com
3D Medicines Forward-Looking Statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
热门文章

请咨询我们










