Clinical Trial
Internal clinical team accelerating trial progress
Identify potential targets
Experienced clinical team focus on high potential targets
Obtain future market-leading drugs
through in-house R&D or licensed in assets
Team collaboration
Global Clinical Operation
Department of Clinical Research
Department of Medicine
Department of Registration
Trial execution
Reasonable and efficient trial planning
Forward-looking design & parallel actions: start the next phase of the process based on anticipation to reduce the total duration
Efficient clinical progress
Envafolimab from Phase I to NDA only takes less than 4 years
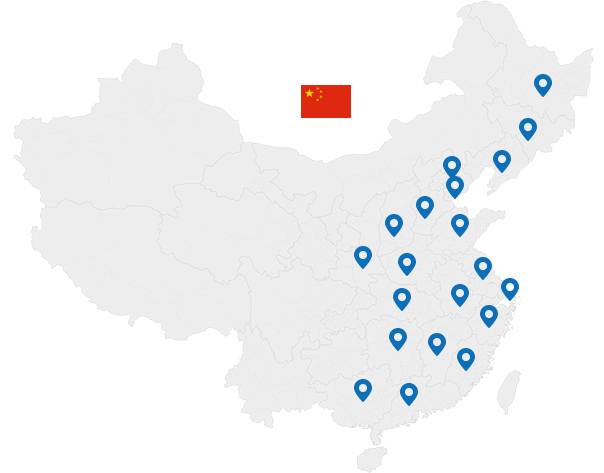
Carrying out clinical trials throughout China and the rest of the world, partnering with top PIs to advance the R&D process
-
70+ cooperative hospitals
-
20+ covered provinces
-
82+ renowned PIs