3D Medicines Inc. Announces that the IND for Phase 1 Clinical Trial to Evaluate 3D189 (Galinpepimut-S), a peptide cancer vaccine, was approved by NMPA
Beijing,April 6,2022 - 3D Medicines Inc. (the “Company”), today announced that an IND application to initiate the first clinical trial in China for 3D189, also known as SELLAS’s galinpepimut-s (“GPS”), has been approved by China’s National Medical Products Administration (“NMPA”) to evaluate safety and immunogenicity of 3D189 in Chinese patients with hematological malignancies.

This is a phase 1, open-label, single-arm, multi-center study to assess the safety and immunogenicity of 3D189,a WT1 peptide vaccine, in patients with acute leukemia in complete response, or patients with multiple myeloma, non-Hodgkin’s lymphoma, or higher-risk myelodysplastic syndrome, who have received at least 1st-line standard therapy and achieved a response.
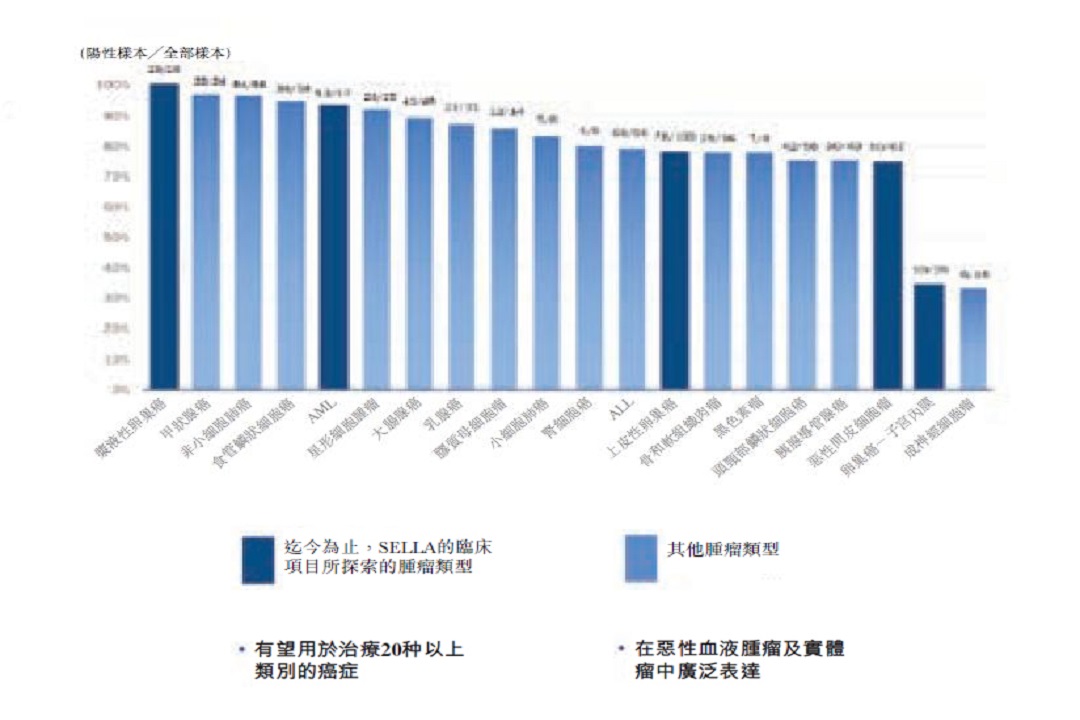
3D189 is a therapeutic cancer vaccine that targets WT1, which was a top ranked antigen for immunotherapy according to the NCI in 2009. As shown in the figure below, WT1 targeted therapies can potentially treat over 20 cancer types (including lung cancer and CRC), which creates a large potential market with a wide coverage of patients.
3D189 induced immune response has the potential to recognize and destroy cancer cells and provide ongoing support and memory to the immune system so that it can continue to target and destroy recurring tumors and residual cancer cells. 3D189 has the potential to be a highly effective approach to prolonging survival by delaying or preventing relapse/recurrence in patients in complete remission or with low tumor burden. In addition, across the five completed studies with 3D189 monotherapy, 3D189 exhibited a highly tolerable safety profile,the only Treatment Related Adverse Events (TRAEs) of any grade that occurred in more than 10% of the patients across all studies were injection site reaction in 18.2% of all patients (Grades 1 and 2 only) and fatigue in 14.9% of all patients (Grades 1 and 2 only).
In previous clinical trials, 3D189 showed promising early anti-tumor activity in patients with AML who achieved second hematologic complete remission, with or without thrombocytopenia (CR2/CR2p),and our partner, SELLAS Life Sciences Group, is conducting a Phase III pivotal clinical trial for 3D189 (GPS) in this patient population in the U.S., Europe.
In December 2020, 3D Medicines obtained exclusive rights from SELLAS to develop, manufacture and commercialize 3D189 in China, Hong Kong, Macau and Taiwan region for all therapeutic indications and other diagnostic uses.
About 3D Medicines Inc.
3D Medicines Inc. is a commercial-stage biopharmaceutical company with a mission to help people with cancer live longer and better. Envisioning a future when cancer is managed as a chronic disease, 3D Medicines focuses on the development of differentiated immuno-oncology drugs, helping cancer patients live with prolonged survival time and a better quality of life. 3D Medicines has established a pipeline with both biological macromolecule and chemotherapeutic small-molecule drugs, as well as a professional team capable of global development, registration and commercialization operation.
About SELLAS Life Sciences Group, Inc.
SELLAS is a late-stage clinical biopharmaceutical company focused on the development of novel therapeutics for a broad range of cancer indications. SELLAS’ lead product candidate, GPS, is licensed from Memorial Sloan Kettering Cancer Center and targets the WT1 protein, which is present in an array of tumor types. GPS has potential both as a monotherapy and in combination to address a broad spectrum of hematologic malignancies and solid tumor indications. The Company is also developing GFH009, a small molecule, highly selective CDK9 inhibitor, which is licensed from GenFleet Therapeutics (Shanghai), Inc. for all therapeutic and diagnostic uses in the world outside of Greater China.
For more information on SELLAS, please visit www.sellaslifesciences.com.
Forward-Looking Statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
热门文章

请咨询我们