3D Medicines Inc. Successfully Listed on the Main Board of HKEX,Stock code:1244.HK
(Hong Kong, December 15 , 2022) A biopharmaceutical company in China for the development and commercialization of next-generation tumor immunotherapy drugs - 3D Medicines Inc. (“ 3D Medicines” or the “Company”, Stock Code: 1244.HK), has successfully listed and commenced dealings on the Main Board of the Stock Exchange of Hong Kong Limited ("Hong Kong Stock Exchange”) today.
China International Capital Corporation Hong Kong Securities Limited and China Securities (International) Corporate Finance Company Limited are the Joint Sponsors, Joint Global Coordinators, Joint Representatives, Joint Bookrunners and Joint Lead Managers.

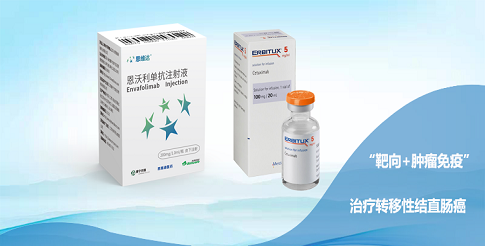
3D Medicines is an oncology-focused biopharmaceutical company with global research and development capabilities. In response to the future trend of chronic treatment of cancer, the Company is committed to developing and commercializing a new generation of oncology immunotherapy drugs with differentiated clinical performance. As of the latest practicable date, 3D Medicines has established a pipeline of one core product and 11 drug candidates, of which the core product, envafolimab (brand name: ENWEIDA, 恩維達®), was approved in November 2021 and commercialized in December 2021 in mainland China. As of May 31, 2022, the sales revenue since the commercialization of the envafolimab was RMB 221 million. And seven other drug candidates are in clinical stage (including those drug candidates for which the Company has initiated clinical trials or has received IND approvals and is preparing for initiation of clinical trials).
A major market player in the field of cancer treatment, especially for patients whoneed long-term care
As one major market player in the chronic cancer treatment market, 3D Medicines has built a complementary pipeline of innovative drug product and drug candidates to cover various stages of drug development and marketing for cancer patients’ need. The Company's core product, envafolimab, is a subcutaneously injectable PD-L1 antibody in 0.75ml (150 mg) dose. Compared to other marketed PD-1/PD-L1 inhibitors, the available clinical data has shown that envafolimab has favorable safety profile and consistent efficacy results in clinical trials as well as a low immune-related pneumonitis rate and no infusion related reactions.
Furthermore, the Company has been building entry barriers in the chronic cancer treatment market by focusing on combinational therapies that treat cancer through complementary mechanisms with potential for synergistic effects. The Company believes this approach will allow the Company to systemically and effectively tackle tumors, and will significantly improve the life quality and survival rates of cancer patients.
A multi-mechanism and highly synergetic pipeline of innovative drugs
3D Medicine’s vibrant chronic cancer treatment ecosystem focus on immuno-oncology therapies whichis supported by cancer-related pain management solutions. As the Company strives to explore and capture oncology market opportunities, through both in-house discovery and external licensingof highly innovative products, the Company has assembled and are developing a portfolio of therapies to help cancer patients who need long-term care. Over the years since the Company’s inception, they have maintained R&D investment, adopted a forward-looking multi-stream strategy and built technological expertise, thus culminating in a pipeline of product and drug candidates that covers multiple therapeutic targets/pathways and employs diverse mechanisms of actions for chronic cancer treatment. The Company’s pipeline products and drug candidates not only show differentiated properties in pre-clinical and/or clinical studies, but also have potential for synergy when used in combination with each other, promising broad clinical application prospects and market potential. Each product and drug candidate can potentially be utilized both as a monotherapy and in combination with other therapies that together may unleash potentially breakthrough efficacy. The Company’s drug candidates may synergize with envafolimab to better guide the human immune system to fight cancer and prolong the survival of cancer patients.
Successful exploration of innovative oncology therapies with resources consolidation, business development, clinical development and registration capabilities
To ensure to maximize commercial value of its product and drug candidates, the Company has also sought strategic collaboration opportunities worldwide. The Company has worked with reputable PIs to carry out various clinical trials to realizethe clinical and commercial value of the commercial product and drug candidates. Besides, the Company has collaborated with TRACON to carry out clinical trials for envafolimab in patients with soft tissue sarcoma in the U.S., Canada and Mexico.Meanwhile, 3D Medicines and Merck Healthcare KGaA (“MRKDG”) entered into a clinical trial collaboration and supply agreement for conducting a Phase II clinical pilot study in Greater China, in which envafolimab and cetuximab (Erbitux®), a monoclonal antibody targeting epidermal growth factor receptor (EGFR) developed by MRKDG, would be dosed in combination.
Since inception, the Company has maintained long-term and effective R&D investment. In 2020, 2021 and the first five months (ended May 31) of 2022, the R&D expenses had reached RMB264.0 million, RMB371.2 million and RMB138.3 million, respectively. As of the latest practicable date of the prospectus, its patent portfolio were consisted of 87 patents/patent applications that were owned/co-owned by or licensed to the Company (including 57 outside of China), including 26 for its Core Product envafolimab.
Full research and clinical development capabilities with proven track record from discovery to NDA stage
The Company’s R&D platform has molecule screening and design capabilities that increase the possibility of success of moving molecules from pre-clinical studies to market, enable innovative therapeutic approaches and support pipeline assets built around key pathways and targets. The Company’s R&D centers in Shanghai and Beijing include large and small molecule platforms, cell line screening platforms, compound screening platforms and animal models. As of the latest practicable date, the Company had obtained 16 IND approvals and implemented 12 Phase II/III clinical studies.
Internationally skilled management and R&D team
The Company specializes in the entire drug R&D process from pre-clinical research to clinical development and to commercialization. The management team has an average of 20 years of industry experience at reputable organizations such as the FDA, BMS, AstraZeneca AND Celgene. Led by Dr. Gong, the Chairman and CEO, its outstanding management team has attracted a number of professionals. As of latest practicable date of the prospectus, the company has 246 employees, with 149 employees in the R&D team and 81 of which have a master’s degree or higher, including 17 with doctor’s degrees. With the management team’s deep industry insights and sound judgment, the Company has built a synergetic pipeline of innovative drug product and drug candidates.
Overall, 3D Medicines has many advantages and is in an unique position in comparison with some other drug companies because of its broad track of various cancer targets, efficient R&D progress, excellent drug pipeline including the drug product and drug candidates, and superior management and technical teams. More importantly, compared to many other biotech companies which are still in the early stage of R&D, 3D Medicines, as its core product, envafolimab, has already been launched on the market, will be supported by continuous cash flow generated from its sales operations in the future, which will provide great support to the R&D platform and achieve a virtuous circle development.

Dr. Zhaolong Gong, Chairman, Executive Director and Chief Executive Officer of 3D Medicines
Dr. Zhaolong Gong, Chairman, Executive Director and Chief Executive Officer of 3D Medicines said, " Today, we are very delighted to witness 3D Medicines to be officially listed on the Hong Kong Stock Exchange. This is an important milestone in our history of development as well as a starting point of our new journey. The market is expected to grow significantly as the number of patients with chronic cancer increases and innovative therapies for cancer treatment continue to be developed at a rapid pace. We believe that with our competitive position in the innovative pharmaceutical industry, we are well-positioned to accelerate the development of new medicines for cancer treatment and capture a greater share of the rapidly growing market. Looking ahead, we are committed to discovering, developing, and commercializing innovative, safe, and effective drugs for the long-term treatment of cancer patients. We will further strengthen our position in this market by implementing the following strategies, including: further expand the commercial potential of envafolimab and explore new market opportunities; accelerate the product development to commercialization process; enhance our in-house innovative R&D capabilities; establish cGMP manufacturing capability; and continue to attract, cultivate, and retain talent. We are committed to continuously creating greater value for shareholders and investors.”
About 3D Medicines, Inc.
3D Medicines Inc. is a commercial-stage biopharmaceutical company with a mission to help people with cancer live longer and better. Envisioning a future when cancer is managed as a chronic disease, 3D Medicines focuses on the development of differentiated immuno-oncology drugs, helping cancer patients live with prolonged survival time and a better quality of life. 3D Medicines has established a pipeline with both biological macromolecule and chemotherapeutic small-molecule drugs, as well as a professional team capable of global development, registration and commercialization operation.
For more information, please visit
Forward-Looking Statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
热门文章

请咨询我们