The Construction of 3D Medicines’ R&D and Manufacturing Center Started in Xuzhou, Jiangsu

February 26, 2021 – the construction of 3D Medicines’ R&D and Manufacturing Center was officially started, which was announced at the Xuzhou venue of the Joint Groundbreaking Ceremony of Jiangsu Province, as one of the 2021 Key Projects in Jiangsu. Present at the ceremony were the officials of Xuzhou, including Zhou Tiegen, Secretary of Xuzhou Municipal Party Committee and Chairman of the Standing Committee of Xuzhou Municipal Party Committee; Zhuang Zhaolin, Vice Secretary of Xuzhou Municipal Party Committee and Mayor of Xuzhou City; Wang Jianfeng, member of the Standing Committee of Xuzhou Municipal Party Committee and Executive Vice Mayor of Xuzhou City; Wang Qiang, member of the Standing Committee of Xuzhou Municipal Party Committee and Secretary-General of Xuzhou Municipal Party Committee; and Zhou Tianwen, Secretary-General of Xuzhou Municipal Government. Located in the Xuzhou Economic & Technological Development Zone, the R&D and Manufacturing Center of 3D Medicines is adjacent to the East Lake Medical Innovation Port praised as “the new landmark of the biopharmaceutical and big health industry”. Covering about 16.5 acres, the R&D and Manufacturing Center will function as a key base for 3D Medicines to achieve integrity of R&D and manufacturing once completed. For 3D Medicines, construction of the R&D and Manufacturing Center in Xuzhou marks a significant step towards a biopharmaceutical company integrating research, production, and marketing.




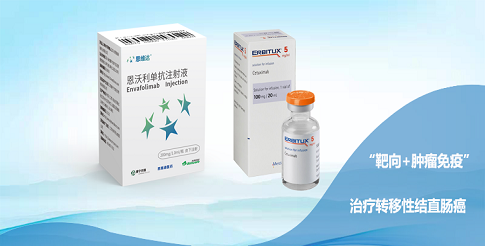
3D Medicines, a biopharmaceutical company focusing on the development and commercialization of innovative immuno-oncology drugs, builds a comprehensive pipeline to seize the trend of cancer chronicization composed of backbone oncology products and complementary pain management solutions, in the purpose of meeting the massive market demand brought by the trend of cancer chronicization. By now, the NDA of Envafolimab, the world’s first subcutaneous PD-L1 antibody innovative drug co-developed by 3D Medicines, has been granted Priority Review by the Center for Drug Evaluation (CDE) under the National Medical Products Administration (NMPA), and the research for multiple innovative drugs of the company have proceeded to late-stage clinical trials, signaling the marketing and profiting at the corner.
Xuzhou has listed the biopharmaceutical and big health industry among four Strategic Emerging Industries; introduced series of policies on technology, talents and marketing services; launched an array of projects in key industries and established platforms including the East Lake Medical Innovation Port. The unique advantages of Xuzhou have attracted lots of outstanding companies. With the strong support of the officials of Xuzhou, the R&D and Manufacturing Center of 3D Medicines has been smoothly sited in the Xuzhou Economic & Technological Development Zone, and listed as a 2021 Key Project in Jiangsu and No.1 Project in Xuzhou.


John Gong, M.D., Ph.D., Chairman and Chief Executive Officer of 3D Medicines, expressed his gratitude for the support provided by the officials of Xuzhou and commented, “For the anti-tumor innovative drug R&D project of 3D Medicines, the whole process from early-stage negotiation to the groundbreaking ceremony today took only three months. Behind the Xuzhou speed we have seen, we could feel the faith and support for the biopharmaceutical industry from the city of Xuzhou as a whole. We believe that 3D Medicines will grow rapidly on the promising land of Xuzhou, contributing to the development of the biopharmaceutical industry in Xuzhou; and laying the foundation for 3D Medicines’ growth into a biopharmaceutical company integrating research, production, and marketing.”


The effect rendering of 3D Medicines’ R&D and Manufacturing Center
About 3D Medicines
3D Medicines, Inc. is a clinical-stage biopharmaceutical company with a mission to help people with cancer live longer and better. Envisioning a future when cancer is managed as a chronic disease, 3D Medicines focuses on the development of differentiated immuno-oncology drugs, helping cancer patients live with prolonged survival time and a better quality of life. 3D Medicines has established a pipeline with both biological macromolecule and chemotherapeutic small-molecule drugs, as well as a professional team capable of global development, registration and commercialization operation.
For more information, please visit: www.3d-medicines.com
Forward-looking Statement
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
热门文章

请咨询我们