Latest Clinical Trial Results for Subcutaneous PD-L1 Antibody Envafolimab(KN035)Published on ASCO 2020 Annual Meeting
Beijing, China, May 29, 2020 - 3D Medicines, Inc.,a clinical stage biopharmaceutical company focusing on the development and commercialization of differentiated next-generation immuno-oncology drugs, today published the latest clinical trial results for Subcutaneous PD-L1 Antibody Envafolimab(KN035)on the 2020 annual meeting of American Society of Clinical Oncology (ASCO), under the title of “Envafolimab (KN035) in advanced tumors with mismatch-repair deficiency”. Among the patients who have completed at least two post-baseline tumor assessments, the objective response rate (ORR) of Envafolimab(KN035)was 30% in 50 patients previously treated with fluoropyrimidine, oxaliplatin and irinotecan, including the patients with MSI-H/dMMR colorectal cancer (CRC) (n=39) and patients with advanced gastric cancer (GC) who have failed systemic treatment at least once (n=11), which is highly consistent with the data of Keytruda and Nivolumab, the PD1 inhibitors approved by the FDA for the same indication.
Envafolimab(KN035), the world’s first subcutaneous PD-L1 antibody, is stable at room temperature, saves the medication time for patients, improves compliance and provides patients with a better quality of life. Compared to all the PD-L1 antibodies in development or on the market, Envafolimab(KN035)has significant advantages in the route of administration, and is the hope for realizing the management of cancer as chronic illness.
Background: This open-label pivotal phase II study evaluated the safety and antitumor activity of KN035 in patients with advanced microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) cancer. Methods: The study is a single-arm, open-label pivotal clinical trial, with the objective response rate (ORR) per RECIST v1.1 by independent radiology review as the primary endpoint. MSI-H/dMMR status was assessed centrally for colorectal cancer (CRC) and gastric cancer (GC) and locally for other tumors. Please see the picture below for more details.
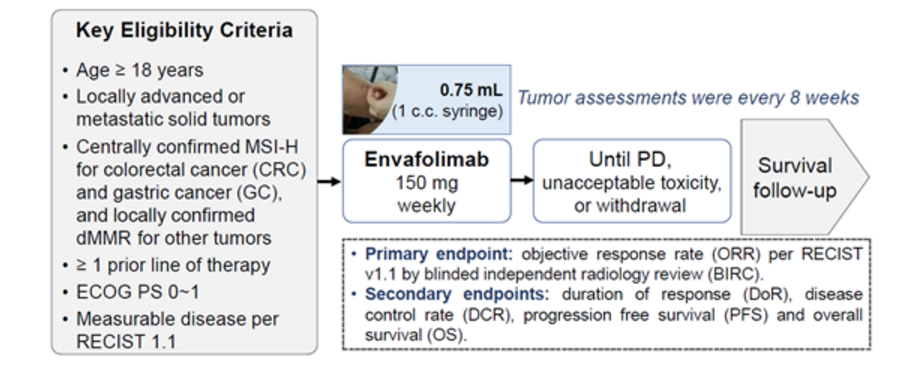
Results: As of December 17, 2019, 103 patients with MSI-H / dMMR advanced cancer were enrolled at 25 centers in China. The primary efficacy population (PEPi) consisted of 39 patients with advanced CRC who had previously received therapies with at least fluoropyrimidine, oxaliplatin and irinotecan and 11 patients with advanced GC who had previously received at least first-line standard therapy, with a median follow-up time of 7.5 months. The overall population (n=103) included 65 patients with CRC (24 previously treated with fluoropyrimidine, oxaliplatin or irinotecan), 18 with GC and 20 with other tumors, with a median follow-up time of 6.7 months. Highlights include:
The confirmed objective response rate (ORR) was 30% (95% CI: 17.9%, 44.6%) in the PEPi, with 80% of those still responding at the time of data cutoff.
The confirmed ORR was 54.2% (95% CI: 32.8%, 74.4%) in the CRC patients previously treated with fluoropyrimidine, oxaliplatin or irinotecan, with 84.6% of those still responding at the time of data cutoff.
The confirmed ORR was 34.0% (95% CI: 24.9%, 44.0%) in the overall population, with 85.7% of those responding at the time of data cutoff.
Key efficacy indicators
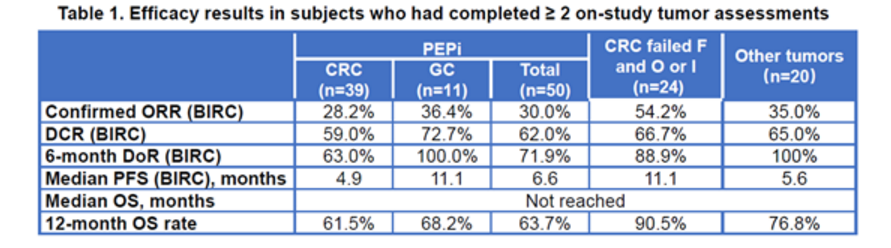
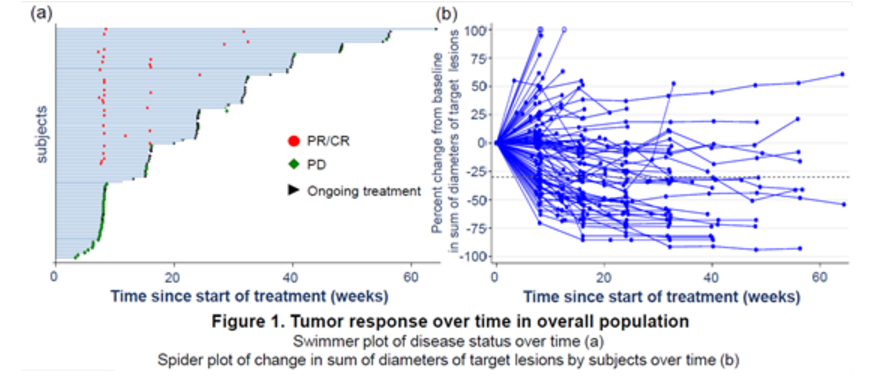
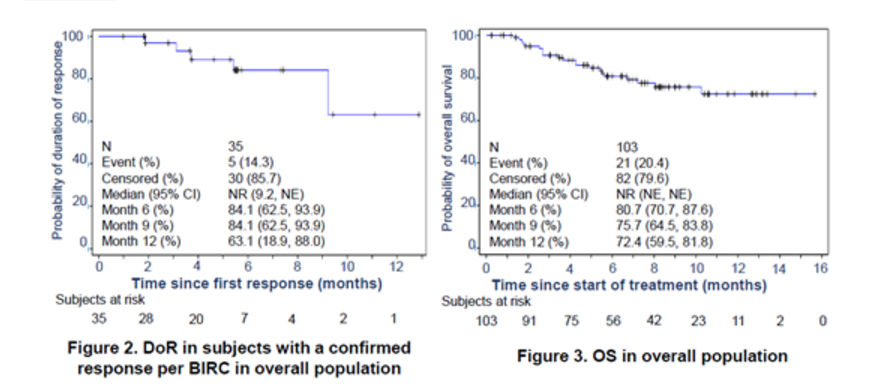
Median progression-free survival was 6.6 months in both the PEPi and the overall population. Median overall survival was not reached in both populations. Grade 3-4 treatment-related adverse events (TRAE) occurred in 14 patients (13.6%). No reports of grade 5 TRAE, pneumonitis, or colitis were reported. Local injection site reaction occurred in 9 patients, all of which were grade 1 or 2.
Drug related Treatment Emergent Adverse Events (TEAEs)
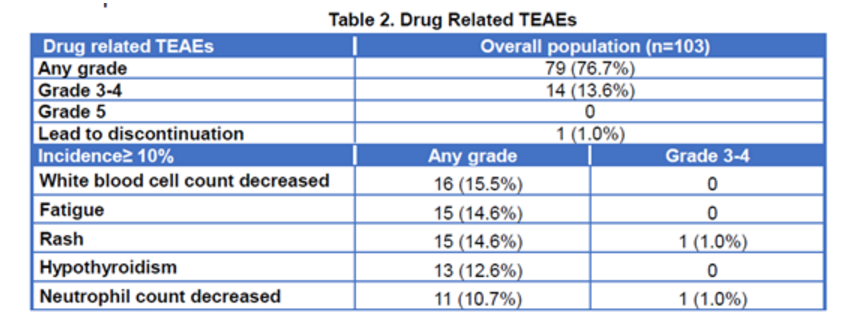
Conclusion: Envafolimab (KN035) demonstrated durable anti-tumor activity with a manageable safety profile in patients with previously treated advanced MSI-H/dMMR cancer.
About KN035
Envafolimab (KN035) is a PD-L1 single-domain antibody Fc fusion protein independently developed by Alphamab. Based on the unique design, Envafolimab (KN035) has advantages in safety, convenience and compliance, and can be used for the patients who are not suitable for intravenous infusion with a lower medical cost. In February, 2016, 3D Medicines and Alphamab signed a collaborative development agreement. Alphamab, as the original research party, is responsible for production and quality, and 3D Medicines is responsible for global clinical development in the field of oncology, registration, global market development and commercialization. On March 30, 2020, Alphamab, 3D Medicines and Simcere reached a three-way strategic collaboration, and Simcere is responsible for the exclusive commercial promotion of the product in mainland China.
At present, Envafolimab (KN035) has been simultaneously tested in clinical trials for multiple cancer indications in China, the United States and Japan, and the research for multiple indications have entered the registration/ clinical Phase III. Envafolimab (KN035) has been awarded Orphan Drug Designation by FDA, and the New Drug Application (NDA) to NMPA is planned to be submitted in 2020.
About 3D Medicines
3D Medicines, Inc. is a clinical-stage biopharmaceutical company with a mission to help people with cancer live longer and better. Envisioning a future when cancer is managed as a chronic disease, 3D Medicines focuses on the development of differentiated next-generation immuno-oncology drugs, helping cancer patients live with prolonged survival time and a better quality of life. 3D Medicines has established a pipeline with both next-generation biological macromolecule and chemotherapeutic small-molecule drugs, as well as a professional team capable of global development, registration and commercialization operation.
About Alphamab Oncology
Alphamab Oncology is a biopharmaceutical company focusing on the research and development, manufacturing and commercialization of biologics for oncology. On December 12, 2019, the Company was listed in the mainboard of Hong Kong Stock Exchange with stock code 9966. Alphamab has fully integrated proprietary biologics platform in bi-specifics and protein engineering. Its highly differentiated in-house pipeline consists of eight anti-cancer drug candidates, four of which have advanced into Phase I – III clinical development phases in China, US and Japan.
The Company also has proprietary CRIB and CRAM platforms for bi-specifics and antibody mixtures, and state-of-the-art manufacturing capability designed and built to meet NMPA and EU/FDA’s cGMP standards. With multiple in-house proprietary platforms for innovative biopharmaceuticals, Alphamab Oncology has built a robust pipeline in oncology/immunology to benefit cancer patients around the world. Visit www.alphamabonc.com for more information.
About Simcere
Simcere is a research and development-driven Chinese pharmaceutical company with a State Key Lab for Translational Medicine and Innovative Drug Development. It is committed to delivering highly effective treatment to patients. Simcere achieves this by focusing its efforts on therapeutic areas of oncology, neurology, inflammation/immunology diseases and more. By leveraging its commercial capability, all of the company’s best products have achieved leading market shares in China. Simcere continues to promote the advancement of international scientific and medical breakthroughs through a collaborative R&D strategy and extensive strategic partnership with several multinational companies.
For more information, please visit: www.simcere.com
Note: (1) The clinical development, registration and commercialization of soft tissue sarcoma in North America is led by Tracon.
热门文章

请咨询我们










