3D Medicines Inc,Announces that the IND for Phase 1 Clinical Trial to Evaluate 3D197 (IMC-002), a of CD47 Antibody Drug, was approved by NMPA
Beijing, January 7, 2022, 3D Medicines Inc, announced that China‘s National Medical Products Administration(NMPA)has approved 3D Medicines’ IND to initiate this first Phase 1 trial with 3D197 in China. 3D Medicines also plans to subsequently conduct a Phase 1b/2 study to evaluate the combination of 3D197 with envafolimab, azacitidine, rituximab, and other standard agents in solid tumors and hematological malignancies.
About 3D197(IMC-002)
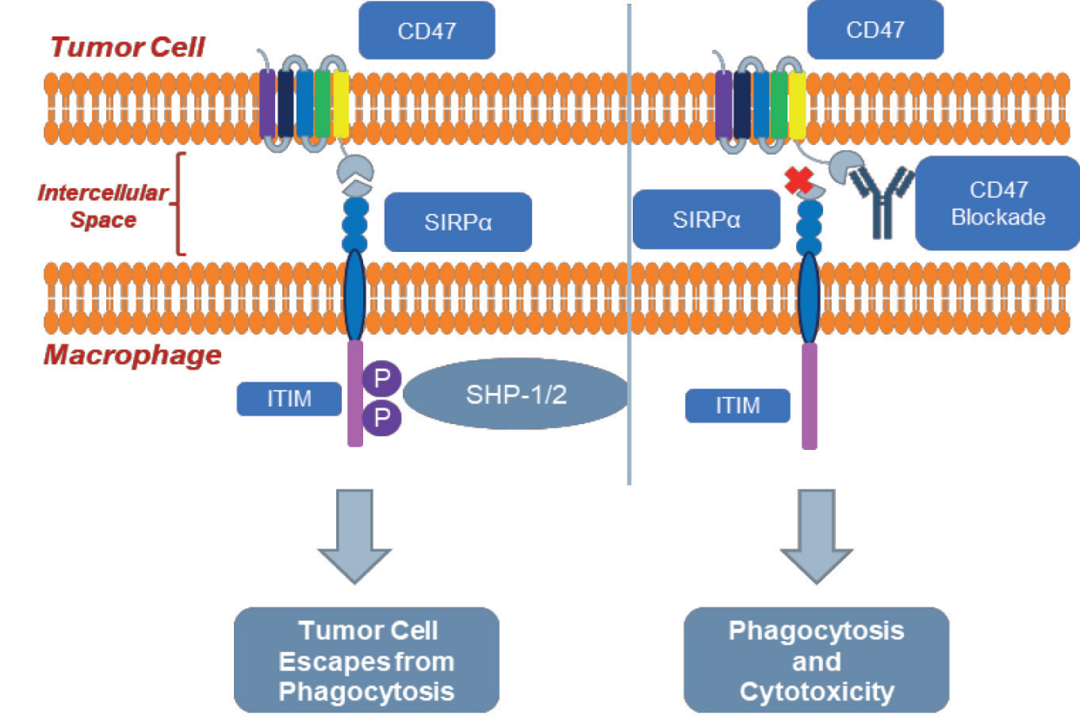
3D197 is a fully humanized anti-CD47 IgG4 monoclonal antibody that blocks the CD47–SIRPα interaction (see diagram for mechanism of action). Results from pre-clinical studies have shown that 3D197 binds to human CD47 with an optimal affinity that maximizes efficacy. 3D197 did not cause hemagglutination in vitro or induced anemia in pre-clinical toxicology studies. 3D197 blocks the “don’t eat me signal,” thereby enhancing tumor phagocytosis by macrophages.
About 3D Medicines, Inc.
3D Medicines, Inc. is a commercial-stage biopharmaceutical company with a mission to help people with cancer live longer and better. Envisioning a future when cancer is managed as a chronic disease, 3D Medicines focuses on the development of differentiated immuno-oncology drugs, helping cancer patients live with prolonged survival time and a better quality of life. 3D Medicines has established a pipeline with both biological macromolecule and chemotherapeutic small-molecule drugs, as well as a professional team capable of global development, registration and commercialization operation.
About ImmuneOncia Therapeutics, Inc.
ImmuneOncia is an immuno-oncology-centric biopharmaceutical company, established in 2016 as a joint venture company between Yuhan Corporation in South Korea and Sorrento Therapeutics, Inc. in the US. In March 2021, ImmuneOncia and 3D Medicines signed an exclusive license agreement to develop, manufacture and commercialize IMC-002 in Greater China. Meanwhile, ImmuneOncia continues to retain the rights of IMC-002 in the rest of the world including the United States, European Union and Japan. A Phase I study of IMC-002 by the company is ongoing in the US and Korea.
Forward-looking Statement
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
热门文章

请咨询我们









